For every gallon of biodiesel synthesized from soybean crops or other sources, more than a pound of waste consisting mostly of glycerol is created. Scientists are finding ways to convert that glycerol into something valuable.
When getting a new car, buyers balance luxuries, performance, and price, and mixed in with all that is the choice of "fuel." Some will consider electric and hybrid cars, a gentler option on the environment, but few will think of diesel as a renewable option. But, it could be.
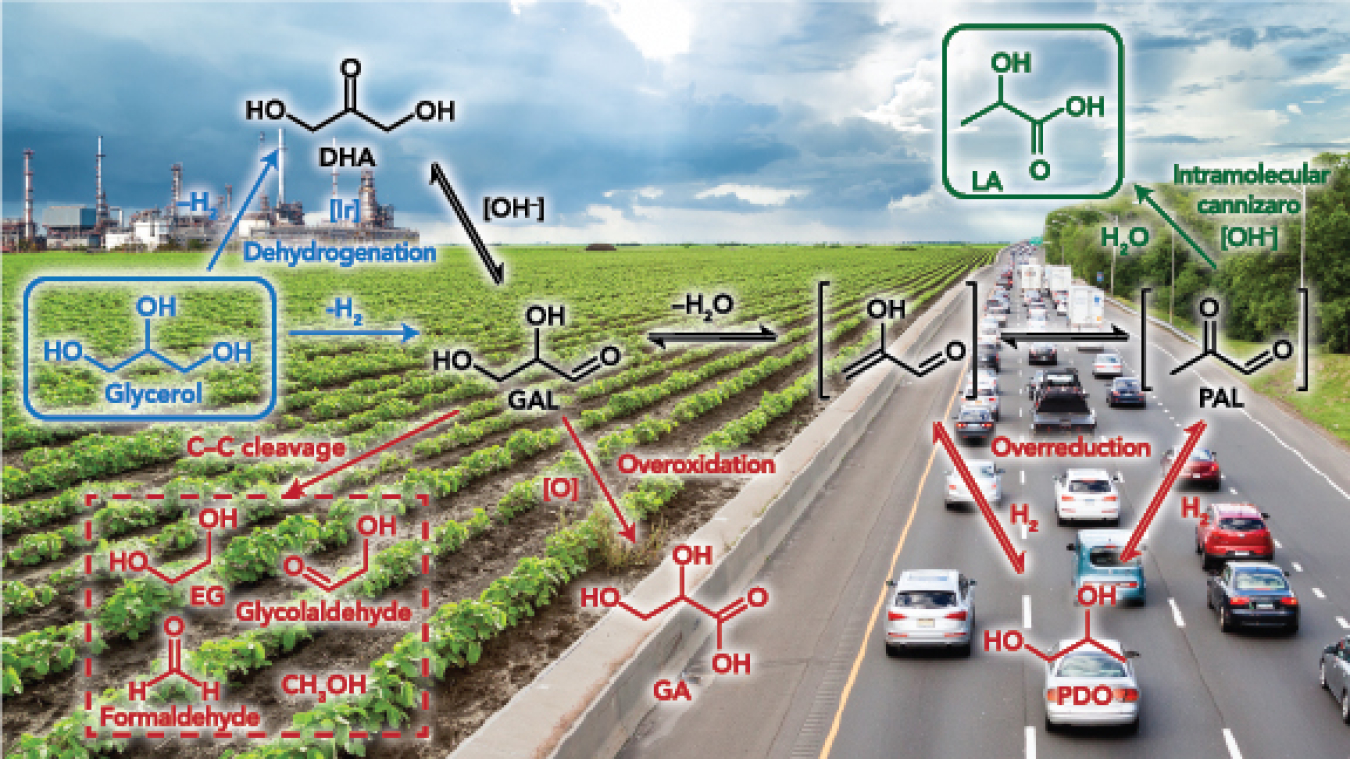
Diesel can be created from oil from soybean crops or restaurant fryers. Cheap, plentiful oils become the base for diesel fuel. The process works, yet most of the nation's diesel fuel still comes from refining crude oil. Why? For starters, biodiesel production creates a lot of waste. In fact, for every gallon of biodiesel synthesized, more than a pound of dark brown waste consisting mostly of glycerol is created, glycerol that must be discarded or sold; it is not valuable. What if glycerol could be transformed into something that was?
While patents and processes striving to answer this question abound, scientist have yet to devise the perfect solution. While biodiesel could by no means entirely replace diesel from petroleum, it could make an important contribution. The ideal solution would reach the sweet spot – quickly and efficiently turning glycerol into a valuable commodity. Scientists funded by the U.S. Department of Energy are now finding new paths to that sweet spot using tools available through the Energy Frontier Research Centers.
Established by the DOE Office of Science's Office of Basic Energy Sciences, the centers speed discoveries by bringing together talented people and giving them the best tools available to understand and manipulate matter on the atomic and molecular scales.
At the Center for Catalytic Hydrocarbon Functionalization, an Energy Frontier Research Center led by the University of Virginia from 2009 through 2014, chemist Robert Crabtree and his team tried a different route to turn glycerol into lactic acid, which is used in shampoos and is a building block for a host of products. Using a new iridium-based catalyst – the first liquid catalyst for this complex conversion – to enhance the reaction produces 10 times more lactic acid than a comparable solid catalyst. It does not need special handling. It works under mild conditions. It works without exotic solvents. Further, it transforms, albeit a bit more slowly, crude glycerol waste from the biodiesel plant.
The team's work on the liquid catalyst appeared in Nature Communications and provided new insight into how to design a liquid catalyst for glycerol conversion. They are now working on an iron-based catalyst that is much cheaper than their iridium catalyst.
Taking a different approach to glycerol conversion, chemist George Huber and his associates coated an electrode with a solid catalyst and "zapped" the glycerol with electricity. Electrons from the electrodes convert slightly more than 90 percent of the glycerol into glyceraldehyde, a valuable commodity chemical. As a bonus, the process, like Robert Crabtree's, pumped out hydrogen gas, a potentially valuable product.
This work was done while Huber and his team were with the Institute for Atom-Efficient Chemical Transformations, a former Energy Frontier Research Center based at Argonne National Laboratory in Illinois.
Other researchers are tackling the problems that confound a host of glycerol-converting processes, one of which is that it uses a lot of "pots." Like a tidy chef, fewer pots means less time cleaning. And, the process of converting glycerol can take up a lot of pots. Chemist Wei Fan and his peers at the Catalysis Center for Energy Innovation, an Energy Frontier Research Center led by the University of Delaware, devised a one-pot reaction that turns glycerol into large quantities of lactic acid under mild conditions. The secret to their recipe is to combine two catalytic reactions on one catalyst. The result? More lactic acid.
It's often been said, "Waste not, want not." But through fundamental research into the conversion of glycerol, that waste may one day be transformed into fuels that we need and products that we want.
The Office of Science is the single largest supporter of basic energy research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information please visit the Office of Science website.
Kristin Manke was a Communications Specialist at Pacific Northwest National Laboratory on detail to the U.S. Department of Energy's Office of Science, kristin.manke@science.doe.gov.
